What is Platelet-Rich Plasma (PRP)?
An Advanced Overview for Clinicians
Biological Basis of PRP
Platelet-rich plasma (PRP) is an autologous blood-derived preparation produced by centrifuging a patient’s own blood to collect a supraphysiological concentration of platelets, suspended in plasma. Platelets contain a range of growth factors, cytokines, and signalling proteins involved in haemostasis, angiogenesis, and tissue regeneration.
The average baseline platelet count in human peripheral blood is ~200,000 ± 75,000/µL. In whole blood, platelets remain in a quiescent state, with an in-vivo half-life of approximately 7–10 days. Upon activation by exposure to collagen, thrombin, or calcium ions, platelets release more than 5200 different proteins, including growth factors, cytokines and exosomes, from their alpha and dense granules, in a controlled, sequenced manner.
The biological profile of PRP is influenced by multiple variables, including baseline platelet count, platelet recovery efficiency, leukocyte inclusion, and final injectate volume. As a result, PRP is not a single uniform product, but rather an autologous preparation that depends on both patient factors and the platelet-concentrating system used.
PRP-Mediated Healing
PRP accelerates and enhances the body’s intrinsic healing ability, particularly in tissues with poor vascular supply or that have a ‘disrupted’ metabolic activity.
The Importance of Platelet Dose
The regenerative efficacy of a PRP or PRF treatment is directly correlated with the dose of functional platelets delivered to the target tissue, (known as the platelet dose). Like most medications, the regenerative potential of the procedure is linked to the platelet dose; and whether it matches the clinical need and treatment are to exert a clinically relevant biological effect.
The Platelet Proteome:
Growth factors, exosomes, cytokines & signalling molecules
Platelets are small, anucleate cell fragments derived from megakaryocytes. Although they do not contain a nucleus, they contain a diverse array of proteins essential for their function in haemostasis, thrombosis, immune response, and wound healing. (Huang, 2021).
The platelet proteome encompasses the complete set of proteins expressed by platelets, which includes approximately 5200 distinct proteins. These proteins encompass surface receptors, cytoskeletal proteins, granule contents, signalling molecules, and coagulation factors.
The comprehensive and dynamic nature of the platelet proteome enables platelets to efficiently perform their roles in maintaining vascular integrity, responding to injury, initiating and sustain tissue regeneration and healing processes. (Huang, 2021)
Reference: Huang, J., Swieringa, F., Solari, F. A., Provenzale, I., Grassi, L., De Simone, I., ... & Heemskerk, J. W. (2021). Assessment of a complete and classified platelet proteome from genome-wide transcripts of human platelets and megakaryocytes covering platelet functions. Scientific Reports
Platelet-initiated Tissue Regeneration
Platelet‑rich plasma (PRP) injection supports tissue regeneration by delivering a concentrated dose of the patient’s own platelets, and their released growth factors, exosomes, cytokines & bioactive proteins directly into the injured tissue, and then sustaining their action over time.
How PRP initiates repair
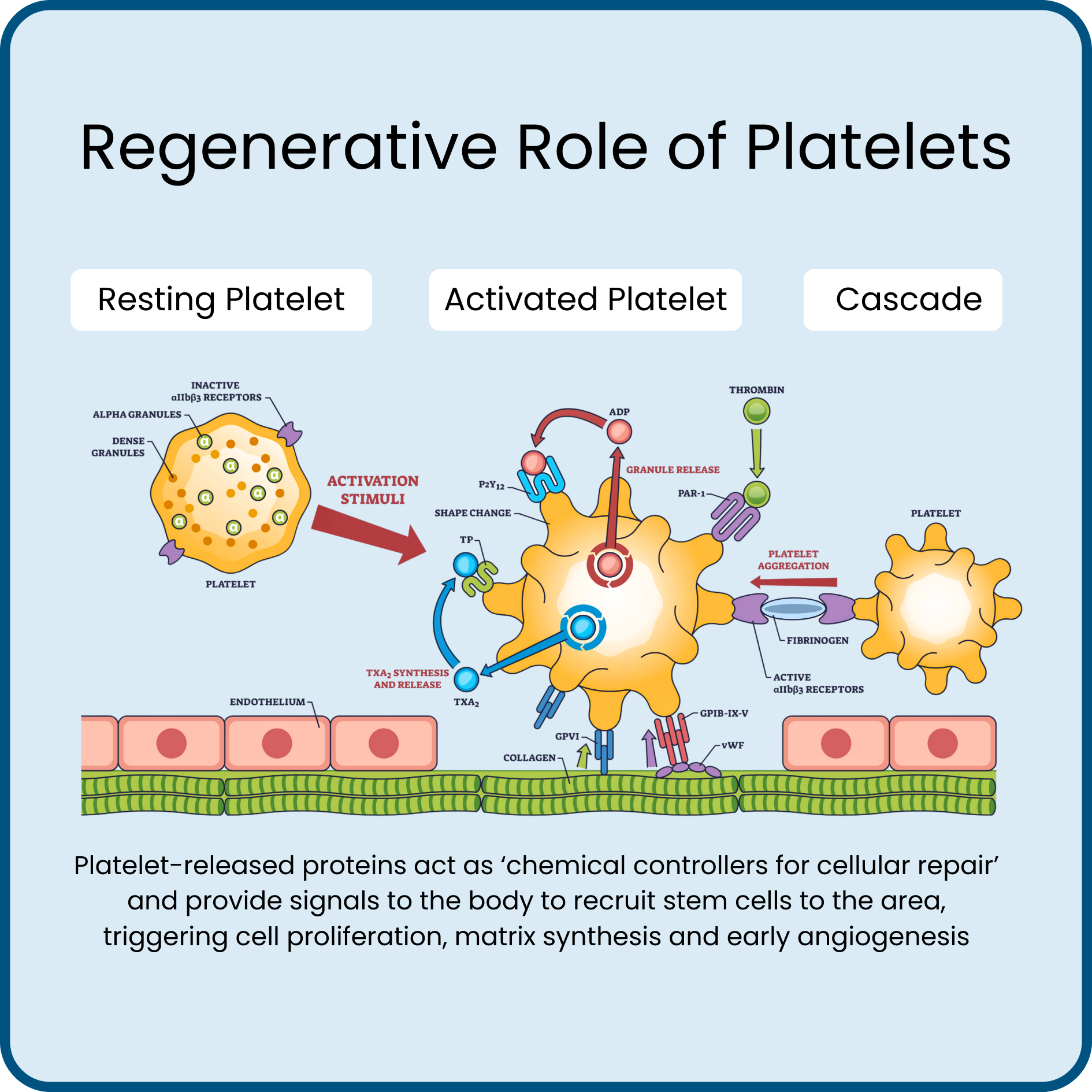
Upon injection, resting platelets adhere to local collagen and are activated, releasing more than 5200 different regenerative proteins from their alpha granules and dense granules.
These proteins active as ‘chemical controllers for cellular repair’ and provide signals to the body to recruit stem cells to the area, triggering cell proliferation, matrix synthesis and early angiogenesis.
PRP also provides an immediate provisional fibrin matrix within the injection site that supports cell migration and organizes early granulation tissue.
How PRP sustains regeneration
Platelet‑released proteins maintain a pro‑healing microenvironment within the injected tissue that helps to modulate inflammation, promote cellular proliferation, and support extracellular matrix reorganisation.
Exosomes and microvesicles from platelets further fine‑tune cell-to-cell communication, influencing angiogenesis, collagen organisation and tissue remodelling.
Why device design matters
Clinically meaningful regeneration depends on delivering a therapeutic platelet dose within a plasma volume that can be fully accommodated within the target site (typically 3-6× baseline platelet concentration is used), and leukocyte content appropriate to the clinical situation.
Variability in density-gradient separation efficiency, red‑cell contamination, and premature platelet activation (during centrifugation before PRP collection) can significantly alter the growth‑factor profile and the balance between pro‑ and anti‑inflammatory chemical signalling, that is delivered to the target site.
Platelet‑concentrating systems that provide reproducible platelet recovery, controllable leukocyte inclusion & predictable output volumes help clinicians deliver a greater regenerative stimulus and provides patients with a better chance of a positive outcome.